Ordering and distribution network
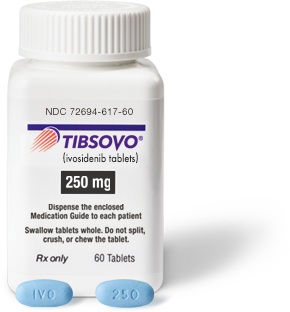
National Drug Code (NDC)
| NDCs |
10-digit code: 72694-617-60 11-digit code: 72694-0617-60 |
| Dosage strength |
250 mg/tablet |
| Description |
250-mg tablet: Blue oval-shaped film-coated tablet debossed “IVO” on one side and “250” on the other side |
The red zero converts the 10-digit NDC to the 11-digit NDC. Some payers may require each NDC to be listed on the claim. Payer requirements regarding the use of NDCs may vary. Electronic data exchange generally requires use of the 11-digit NDC.
Product information
How TIBSOVO is supplied: 250-mg tablets, supplied in 60-count bottles (30-day supply) with a desiccant canister
Storage: Store at 20 to 25 ºC (68 to 77 ºF)
Distribution network for TIBSOVO
TIBSOVO is only available through our specialty distributors and network specialty pharmacies.
Specialty distributors
TIBSOVO is available through specialty distributors for shipment directly to office- or hospital-based pharmacies.
McKesson Specialty Health
Cardinal Health Specialty Pharmaceutical Distribution (US)
Cardinal Health (Puerto Rico)
ASD Healthcare
Oncology Supply
Network specialty pharmacies
TIBSOVO ships directly from the specialty pharmacy to your patient’s home or preferred location.
Biologics by McKesson

Patient support services
ServierONE offers patients helpful resources and tools for navigating treatment care, costs, and education throughout their journey.
Support with insurance coverage and reimbursement
Financial assistance to help patients pay for TIBSOVO
Prescription fulfillment through our network of specialty pharmacies and distributors


